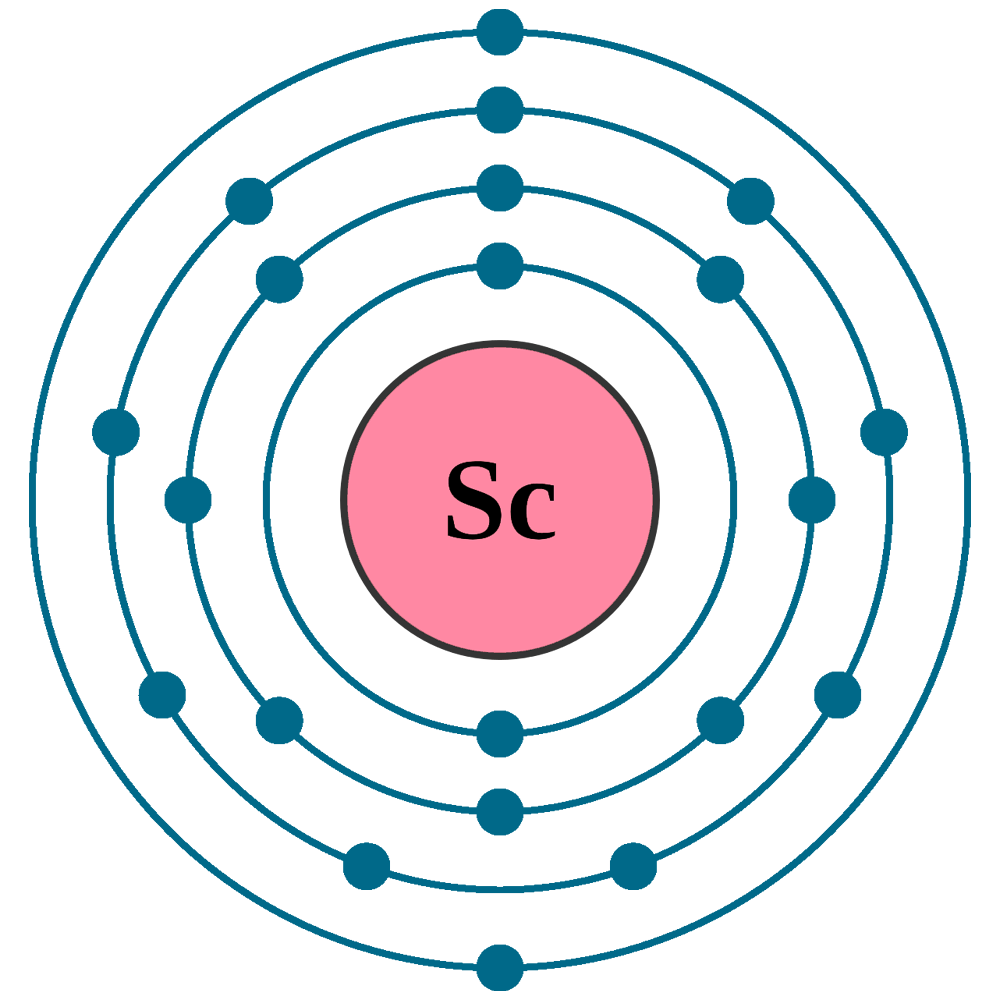
Atomic Number of Scandium Atomic Number of Scandium is 21. Chemical symbol for Scandium is Sc. Number of protons in Scandium is 21. Atomic weight of Scandium is 44.955908 u or g/mol. Melting point of Scandium is 1539 °C and its the boiling point is 2832 °C. A few important properties of scandium are that the mass number of this element is 44.96 u. Scandium belongs to Group 3, Period 4, and d-block of the periodic table. The atomic structure of scandium consists of 21 protons and 24 neutrons. Scandium also displays properties of electronegativity as it’s a metal. What is the atomic no. Of scandium is 21, it is represented by Sc. Lars Nilson have discovered it in 1879. Atomic Mass os Scandium is 44.95591 amu, it have 21 protons/electrons, Hexagonal is its Crystal Structure & color is silvery. Here is its atomic structure.
Atomic Number of Scandium is 21.
Scandium is a chemical element with symbol Sc and atomic number 21. Scandium-45 atom is the stable isotope of scandium with relative atomic mass 44.955910, 100 atom percent natural abundance and nuclear spin 7/2. It is a soft silvery metal that has three times the density of water and can be found in the form of solid at room temperature. Scandium (Sc) Atomic Data for Scandium (Sc) Atomic Number = 21 Atomic Weight = 44.955910 Reference E95: Isotope: Mass: Abundance: Spin: Mag Moment: 45 Sc.
Chemical symbol for Scandium is Sc. Number of protons in Scandium is 21. Atomic weight of Scandium is 44.955908 u or g/mol. Melting point of Scandium is 1539 °C and its the boiling point is 2832 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery Year
Atomic Number Of Scandium
About Scandium
How Many Electrons Are In Scandium
Scandium is a rare metal known also as a transition metal. In its pure form, this metal is quite soft and reactive, especially with water and air. It is a carcinogen and quite a toxic element. Its name is derived from a Latin word meaning Scandinavia. Scandium is rather used for research since it is very rare and can be found only is a few rare types of minerals. As a metal, scandium has very low density, so it is used in alloys with other light metals like aluminum in aircraft industry, etc. Combined with mercury and iodine, scandium is used for producing bulbs.
Properties of Scandium Element
| Atomic Number (Z) | 21 |
|---|---|
| Atomic Symbol | Sc |
| Group | 3 |
| Period | 4 |
| Atomic Weight | 44.955908 u |
| Density | 2.989 g/cm3 |
| Melting Point (K) | 1814 K |
| Melting Point (℃) | 1539 °C |
| Boiling Point (K) | 3109 K |
| Boiling Point (℃) | 2832 °C |
| Heat Capacity | 0.568 J/g · K |
| Abundance | 22 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Transition metal |
| Electronegativity (Pauling) χ | 1.36 |
| Ionization Energy (eV) | 6.5615 |
| Atomic Radius | 160pm |
| Covalent Radius | 144pm |
| Valence Electrons | 2 |
| Year of Discovery | 1879 |
| Discoverer | Nilson |
What is the Boiling Point of Scandium?
Proton Number Of Scandium
Scandium boiling point is 2832 °C. Boiling point of Scandium in Kelvin is 3109 K. Mac pages download for free.
What is the Melting Point of Scandium?
Scandium melting point is 1539 °C. Melting point of Scandium in Kelvin is 1814 K.
How Abundant is Scandium?
Abundant value of Scandium is 22 mg/kg.
What is the State of Scandium at Standard Temperature and Pressure (STP)?
State of Scandium is Solid Adobe reader for mac os x free download. Adobe photoshop lightroom 4 free download for mac. at standard temperature and pressure at 0℃ and one atmosphere pressure.
How Many Shells Does Scandium Have
When was Scandium Discovered?
Scandium was discovered in 1879.
Melting Point Of Scandium
